|

|

How Sweet It Is!
A Colorful Sugar Solution Density Column
|
Developers:
| |
|
Grade Levels:
|
|
|
Discipline:
|
Physical Science
|
|
Objectives:
|
- Students will learn the scientific method.
-
Students will learn how to measure the density of liquids.
|
|
Vocabulary:
|
|
|
Background:
|
The scientific method is a cyclic process of observation, experimentation, hypothesis, prediction based on that hypothesis, and further experimentation to test the prediction. One of the most important features of the scientific method is that, if carefully obtained, data generated by experiments can allow predictions of what has gone on in the past and what may go on in the future. The reliability of such predictions depends directly on the accuracy of the data on which it is based. In this experiment, we will examine some of the interactions between measurement, data and prediction in science. While the system we will use for this study is simple, the implications are quite profound.
This experiment will use the scientific method to study the densities of a series of liquids. Density is defined as mass of a sample divided by its volume; we would express this in a scientific equation as
|
Density =
|
mass of sample
volume of sample
|
Equation (1)
|
We use the gram (g) as our unit of mass and the milliliter (mL) as our unit of volume. We measure the mass of a sample with a balance; we measure the volume of a liquid with a graduated cylinder or a volumetric flask.
If you have ever placed an ice cube in water, you are familiar with the concept of relative density; ice floats on water because the density of ice is less than that of water. On the other hand a penny sinks in water because the density of the penny is greater than that of water. Oil is a liquid that is less dense than water; oil will float on the surface of water. Glycerin is a liquid that is more dense than water; water will float on the surface of glycerin.
Each investigative team will use qualitative observations to rank a set of liquids selected from the series in order of increasing densities. All teams will collaborate to predict the rank of all liquids of the series in order of increasing density and each team will test the predicted ranking. Each team will use a quantitative method to determine the value of the density of one of the liquids and the class will collaborate to compare the results of the qualitative and quantitative observations.
|
|
|
|
|
|
|
|
Materials:
| -
transparent drinking straws
-
small beakers - seven(7) for solution preparation plus three (3) for each team
-
top-loading balances
-
variety of volumetric glassware
-
sucrose (table sugar), approximately 300 g (Note that you can use salt instead)
-
water, distilled or deionized, approximately 1 liter
-
food coloring (red, yellow, green and blue)
-
sepatory funnel, 500 mL
-
glass tubing
-
rubber tubing
-
ring stand
-
ring clamp
-
stirring rods
|
|
Solution Preparation:
|
- Prepare seven equal volumes of sugar-water solutions from sucrose (or salt), water and food coloring according to the ratios (by weight although alternate would be to use number of sugar packets) indicated below:
|
COLOR
|
COMPOSITION
|
DYE RATIO
| |
Red
|
100% Water (0% Sugar)
|
Only Red Dye
| |
Orange
|
10% Sugar in Water
|
1: 1 Red:Yellow
| |
Yellow
|
20% Sugar in Water
|
Only Yellow Dye
| |
Green
|
30% Sugar in Water
|
Only Green Dye
| |
Blue
|
40% Sugar
|
Only Blue Dye
| |
Indigo
|
50% Sugar
|
1:2 Blue:Red
| |
Violet
|
60% Sugar
|
1:1 Blue:Red
|
-
Make the solution sets for the teams by placing the colored solutions in separate beakers.
|
|
Procedure:
|
- Each team receives a set of three beakers, each containing a different colored solution, as follows:
-
Team 1: red, blue, yellow
-
Team 2: red, blue, green
-
Team 3: red, blue, orange
-
Team 4: blue, yellow, orange
-
Team 5: blue, yellow, green
-
Each team layers the (pairs of) colored solutions by:
-
dipping a straw into one of the solutions to a depth of about 1 centimeter (about 1/2 inch);
-
putting a finger over the end of the straw;
-
removing the straw from that solution;
-
lowering the straw into the next solution about 2 cm (about 1 inch);
-
remove the finger from the top of the straw (and allow the next layer to move into the straw);
-
put finger over the top of the straw again; and
-
lift straw out vertically.
It is important to keep straw vertical at all times; if straw is slanted the solutions will mix regardless of their relative density.
-
Determine if the solutions have mixed or not.
-
If there are two distinct layers you have the more dense solution on the bottom.
-
If the solutions have mixed, the more dense solution was on top. Repeat the procedure in reverse order to get two distinct layers.
-
Using this method, team should be able to obtain three distinct layers with the least dense layer on the top.
-
After each team has layered their three solutions, the entire class will pool their data to predict the correct order for all five solutions (red, blue, yellow, green, orange).
-
Each team tests the class prediction by layering all five solutions to determine the relative densities of the set of solutions.
-
Each team is assigned to determine quantitatively the density of one of the five solutions.
-
The team will choose one of the available volumetric glassware.
-
The team adds the assigned solution to the container.
-
The team determines the mass of the solution in the container.
-
The team determines the volume of the solution in the container.
-
The team uses Equation (1) to determine the density of the solution.
-
Each team reports the value of the density of the assigned solution to the rest of the class. The class compares the quantitative and qualitative rankings of densities and will attempt to explain any discrepancies between the results of their observations and measurements.
|
|
Demo:
|
- Construct the apparatus according to Figure 2. Be sure to keep the glass tubing very near the bottom of the graduated cylinder during the addition of solution.
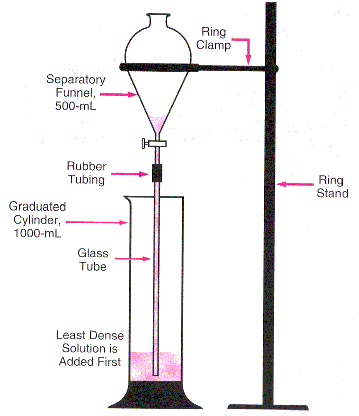
-
Add the red colored water to the separatory funnel. (Regardless of the colors selected, remember to add the least dense solution to the cylinder first!)
-
Partially open the stopcock valve and allow the red solution to slowly drain into the bottom of the graduated cylinder. Close the valve before all the red (least dense) solution drains from the separatory funnel. A continuous volume of solution must be present in the tubing during the entire column "construction".
-
Now add the orange (second least dense solution to the separatory funnel. Close the valve just before all the orange solution drains from the separatory funnel.
-
Add each remaining solution in a similar fashion.
-
Carefully withdraw the glass tube.
|
|
Explanation: |
The greater the amount of sugar in the solution the greater the density.
|
|
Data Record
Date
Team Members:
Solution Set Assigned:
Qualitative Ranking of Solution Densities
List the density of the solutions in your set in increasing order:
List the density of all solutions in increasing order as predicted by the class:
List the density of all solutions in increasing order as determined by qualitative experiment:
Quantitative Ranking of Solution Densities
Assigned Solution:
1. Volume of solution measured ______ mL
2. Mass of container + solution ______ g
3. Mass of empty container ______ g
4. Mass of solution ______ g
5. Density of solution ______ g/mL
|
|
Class results for density of all solutions
Red
Blue
Orange
Green
Yellow
List the density of all solutions in increasing order as determined by quantitative experiment:
|
|
|
|
|
|
|
|
|
Go to Easy and Interesting Science Experiments for the Classroom Grades K-12 ONLINE
Go to the SCIBUS Main Page
Orlando Rainey, Webmaster
|