White glue (like Elmer's®) contains a chemical called poly vinyl acetate. You may have heard of vinyl plastics. Shower curtains and "Saran" food wrap are made of vinyl plastics. The word plastic means something can be shaped or molded easily.
Most of the glue is water. The tiny plastic particles are dispersed or suspended in the water. When the white glue dries, the water evaporates into the air, and the solid plastic particles come together and stick to each other and to certain other things that the glue is next to. The poly vinyl acetate particles in white glue are actually coated with another chemical called poly vinyl alcohol that keeps them from sticking to each other in the bottle and helps keeps them suspended in the water.

Chemists call mixtures, like the white glue, colloids. Mayonnaise is also a colloid. Mayonnaise is made from egg yolks, vegetable oil and water. The egg yolk coats droplets of the oil and keeps the oil suspended or held up in the water so the oil and water do not separate.
The Chemistry of Polymers
Poly is prefix that means many. Polymer means many parts. Pmolecules that are like long chains with many links made of smaller molecules. Molecules are groups of atoms.
Polymers are very important substances. Wood, starch, rubber, cotton, wool, spiders' webs, silk, hair, leather, muscles and fingernails are all examples of polymers that are made in nature. Man-made polymers, or synthetic polymers, are materials such as polyester, polystyrene, polyethylene, teflon®, nylon® and the material in the white glue. Fibers in clothing, carpets and fishing line are all polymers.
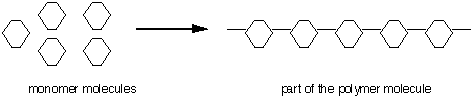
All polymers contain many parts and are made from monomers. The prefix mono means one. Monomer means one part. The monomers link up like a zipper to form the polymer. Polymers may contain hundreds and thousands of parts. Chemists use symbols and models to simplify writing the structures. The diagram shows only a few of the hundreds of parts in a polymer. The arrow is used to show the starting materials that are on the life side are changed to the products on the right side. The arrow can be read as "produce", "go to", "change" or "yields".
The Chemistry of the Cross-Linker
The cross-linker for our experiment is the solution of borax and water (with a little food coloring). Sodium tetraborate decahydrate is the chemical name for borax. It's chemical formula is
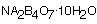 . Na is the symbol for the element sodium; B is the symbol for the boron; and O is for oxygen. Na comes from the word natrium, which is the old Latin name for sodium. The numbers in the formula mean that there are 2 sodium, 4 boron and 7 oxygen atoms combined to make borax. But you have to pour it out of the box to get borax. The 10
. Na is the symbol for the element sodium; B is the symbol for the boron; and O is for oxygen. Na comes from the word natrium, which is the old Latin name for sodium. The numbers in the formula mean that there are 2 sodium, 4 boron and 7 oxygen atoms combined to make borax. But you have to pour it out of the box to get borax. The 10 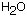 means that there are 10 water molecules attached to each borax. Deca is a prefix meaning ten and hydrate means water.
means that there are 10 water molecules attached to each borax. Deca is a prefix meaning ten and hydrate means water.
The cross-linker is a solution of borax mixed and dissolve in water. Solutions flow just like the water that they are made from, but colloids and gels do not flow like water.
The Molecular Net
When the cross-linker is mixed with the glue and water, chemical bonds break and new bonds form and the bonds link the molecules together. This cross-link forms a network and traps the water in the molecular net. The Goofy Gel is mostly water, but the cross-links make it more rigid and keep the gel from flowing like water.
For more information on the Borax - water chemistry please go to our It's The Slime Time of Your Life experiment.
Cross-links also occur, in a similar way, during hair permanents to make the hair curly. Blood clots occur when protein fibers in the blood stick to each other by cross-linking.