|
Developer:
|
Denise Anderson
Waste Management, Inc.
Oakbrook Laboratory
Geneva, IL
|
|
Grade Levels:
|
K through 12
|
|
Discipline:
|
Physical Science / Chemistry
|
|
Goals:
|
Students will learn about paper chromatography, a technique to separate substances in a mixture.
|
|
Background:
|
Chromatography is a technique used for separating substances in a mixture. In all variations of chromatography, a substance is placed onto or into a medium and a solvent is passed through the medium. As the solvent, which is known as the mobile phase, passes through the medium, some of the substances may be attracted to the solvent and follow it up the medium. Different types of of molecules or substances are transported different distances, causing them to separate. Each different mixture will produce its own pattern. This picture is called a chromatogram.
In this activity, strips of coffee filters serve as the medium, water is the solvent, or mobile phase, and 8 unknown liquid mixtures will serve as the test substances.
Chromatography is used by the environmental chemist to identify possible environmental contaminants in complex mixtures. Sophisticated analytical equipment allows identification and quantification of these contaminants at the parts per billion level (ppb).
|
|
Teacher's Notes:
|
The following is a way to introduce this experiment to students.
Let's imagine that you are an analytical chemist working at environmental lab XYZ. You have just received 8 liquid samples from various landfills for analysis using chromatography. You are looking for environmental pollutant blue. If any of the samples you analyze contain pollutant blue, you are to notify the landfill, so that corrective remedial action can be taken to avoid this pollutant getting into the groundwater system.
|
|
Materials:
|
-
coffee filters - 8 strips of coffee filter paper (2 cm x 10 cm)
-
2 clear 10 oz. cups
-
water
-
magnifying glass
-
*8 unknown liquids: a variety of concentrated Kool-Aid® mixtures in warm water ( 5 mL water / package)
-
toothpicks
-
2 pencils
-
metric rulers
-
graph paper
-
transparent tape
* Use unsweetened Kool-Aid®. If you use Kool-Aid® with xanthan gum or cellulose gum the components will not separate or migrate.
|
|
Procedure:
|
-
Obtain 8 strips of coffee filters. Label each section at the top 1 through 8. Also, put your last name at the top of the strip.
-
Draw a horiztontal line 2.0 cm from the bottom of the strip. This line is known as the line of origin.
-
Using the tip of a toothpick, a dot of one of the test solutions is placed as a spot in the center of the line of origin. Repeat the dotting process several times (approximately 5 - 8). Blow on the spot to hasten drying. Keep the dots small. On the work sheet provided, write down the ID of the test solution.
-
Repeat Step (3) for the other 7 test solutions using a separate strip for each solution.
-
Add water to a 10 oz. cup to just barely cover the bottom. Use transparent tape to hang the first four strips from your pencil. Place the pencil over the cup so that all 4 of the marked strips fit into the cup. Adjust the water, so that the ends of the level strips are just barely into the water. Be certain that the dot you put on the filter is above the water line. Let them hang in the water until the water line has moved up most of the height of the strip. Now using the second cup repeat this same activity using the last four strips. NOTE: It is important that the strips stand freely and are not stuck tight up against the walls of the cup or stuck to each other.
-
When the solvent has separated the mixture into its various components, remove the strips from the plastic cup. Mark, with another pencil how far the solvent (water) traveled up the plate. This is called the flow line. Put this value on the worksheet.
Allow the filter to dry.
-
Using a ruler, measure the distance each color traveled from the point of origin. Measure from the middle of the original spot to the point where migration ended for that color. Repeat this process for each color seen on the strip / chromatogram. Use a magnifying lens to see if you can detect the presence of any faint pigments.
|
|
Worksheet:
|
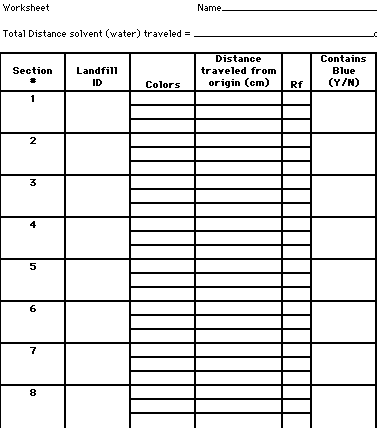
|
|
Results:
|
Develop a histogram for each liquid using the graph paper provided.
-
On the Y axis record the distance traveled.
-
On the X axis record the color observed in the order they appeared, starting from the line of origin.
|
|
Calculations:
|
Compute the retardation Rf values for each color component.
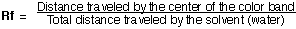
|
Note:
|
Chemist use Rf values to determine which solvent results in the best separation. You must beware, these results vary with experimental conditions. The substrate as well as type of solution effects this value. Mobile phases that give Rf values in the range of 0.2 to 0.8 are best.
|
|
|
Conclusions:
|
After examining your results, which landfills will receive notification that their test sample contains pollutant blue.
The following landfills were notified that there test samples contained pollutant blue:

|
|
Discussion:
|
The degree to which a given substance binds, or sticks to the coffee filter and how well it dissolves in the mobile phase (water) determines how far up that component will move on the filter. A substance that does not bind tightly to the filter or substrate and is highly soluble in water will end up near the top of the plate. The substance that binds to the filter more tightly and is less soluble will be located near the bottom.
|