|

|

And They're Off
|
Developers:
|
Great Ideas from DuPont Teaching Fellows
Compiled by the Kinston, NC
DuPont Communication Team
March, 1994
|
Charles Scaife
Department of Chemistry
Union College
Schenectady, NY 12308
|
|
|
Grade Levels:
|
6 through 8
|
|
Discipline:
|
Physical Science/Chemistry
|
|
Goals:
|
-
Study the chemical reaction of Alka-Seltzer® tablets in water.
-
Determine how long it takes an Alka-Seltzer® tablet to dissolve at different temperatures.
-
Study several other factors which affect the rate of dissolving and reaction of Alka-Seltzer® tablets.
|
|
Background:
|
I have not seen this experiment as given discussed elsewhere, but the same chemical reaction is used commonly, for example, by Andy Sae (1).
The rates of chemical reactions (the speed with which chemical reactions occur) are dependent on a number of factors. One very important factor is temperature. In this experiment each student will determine the time it takes for the components in an Alka-Seltzer® tablet to dissolve and react at a particular temperature.
|
|
Teacher's Notes:
|
The primary difficulty in this experiment is determining the point at which reaction has ceased. Either the disappearance of white solid or the cessation of bubbling or fizzing can be used as a stopping point. The former occurs at shorter times than the latter. Theoretically the cessation of bubbling marks the end of the reaction, but either can be justified. The important thing is to be consistent and have all students perform the experiment in the same fashion.
Plastic beakers are safer, but glass is preferable because of better visibility. CAUTION: The teacher should pour any boiling water from the percolator. Be sure students can safely hadle hot tap water if that is used.
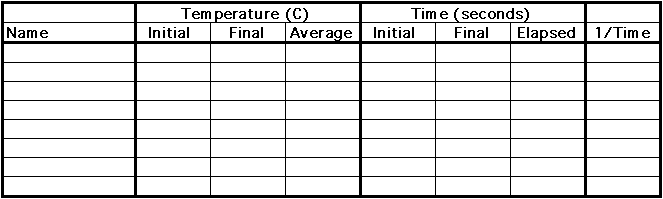
Prepare several data sheets of the form shown.
At the end of the experiment, after each student has tried a different temperature, you should get the class to plot the data in various ways to see if there are any obvious visual relationships between the time required for the reaction and temperature. You might plot
-
Time on the vertical axis versus Temperature on the horizontal axis,
-
the reciprocal of Time (1/Time) [which is closer to representing the reaction rate] versus Temperature,
-
log of (1/Time) [if students have already studied logarithms] versus Temperature,
-
or any other parameters that students want to try.
I like to have pieces of labeled graph paper prepared ahead of time so that students can plot data as soon as they obtain it. These plots can be done manually on pieces of graph paper, but this is also a good opportunity to show the ease and speed of graphing capabilities of a computer if a computer and a graphing program are available.
|
|
Materials:
|
-
3 250-milliliter Beakers
-
3 Thermometers (Celsius scale)
-
Clock or watch with a second hand
-
1 Alka-Seltzer® tablet for each student in the class
-
Pitcher of ice cold water
-
Pitcher of room temperature water
in the class
-
Percolator of boiling water or hot tap water
-
Stirring stick (wooden skewer, wooden splint, or even a straw)
|
|
Explanation:
|
The primary active ingredients in Alka-Seltzer® according are aspirin (an analgesic, that is, a medication that reduces or eliminates pain), sodium acetylsalicylate (also an analgesic) , sodium citrate (an antacid, that is, a substance that neutralizes acids), as well as sodium bicarbonate and citric acid. The latter two substances do not react when kept dry in the tablet form, but do dissolve and react in water solution to produce the fizz of bubbling carbon dioxide gas according to the following chemical reaction.
sodium bicarbonate + citric acid ---> carbon dioxide + water + sodium citrate
Assume that the reaction is complete when you can no longer see any of the white Alka-Seltzer® tablet or when the fizzing from the carbon dioxide formation stops, whichever is specified by the teacher.
|
|
Questions:
|
- Does the rate of dissolving and reaction of an Alka-Seltzer® tablet increase or decreasse as the temperature is increased?
-
Does the temperature of cold water increase or decrease as the dissolving and reaction of an Alka-Seltzer® tablet proceeds? Why?
-
Does the temperature of hot water increase or decrease as the dissolving and real time. When the Alka-Seltzer® tablet has dissolved completely or ceased fizzing from production of carbon dioxide, whichever is designated by your teacher, note the time, and record it on the data sheet as final time. Check the temperature again at the action of an Alka-Seltzer® tablet proceeds? Why?
-
Does the rate of dissolving and reaction of an Alka-Seltzer® tablet increase or decrease at a given temperature if the mixture is stirred rapidly during the process? Why?
-
Does the rate of dissolving and reaction of an Alka-Seltzer® tablet increase or decrease at a given temperature if the Alka-Seltzer® tablet is first crushed before placing it in the water? Why?
-
Are reactions in our body speeded up or slowed down when we have a fever the form shown.
|
|
References:
|
- Andy Sae, Chemical Magic from the Grocery Store, Eastern New Mexico University, Portales, NM, 1991, pp. 26-27, 30-31, and 79-80.
|
|
Procedure:
|
Use hot and/or cold water from the faucet or the appropriate pitchers to adjust the temperature of the water to whatever temperature you desire (as measured on the Celsius scale on the thermometer provided). Be certain that you obtain a temperature for your water that is no closer than 2° C to the temperature used by any previous experimenters as shown on the data sheet. Somebody should be sure to use the hottest water that can be obtained from the faucet or the percolator. Somebody else should be sure to use the coldest water that can be obtained from the faucet or the pitcher of ice water. Add water into the glass beaker provided until it reaches the 100-milliliter mark.
Write your name on a data sheet. Obtain one Alka-Seltzer® tablet. Check the temperature of the water in you beaker once again, and record it on the data sheet as the initial temperature. As you add hte tablet to the beaker, note the time, and record it on the data sheet as the initial time. When the tablet has dissolved completely or ceased fizzing from the production of carbon dioxide, whichever is designated by your teacher, note the time, and record it on the data sheet as final time. Check the temperature again at the end of the reaction, and record it on the data sheet as the final temperature. Determine an average temperature from the one at the beginning and the one at the end of the time, and record it. Also record the elapsed time for dissolving and reaction.
One student should repeat the above procedure at room temperature except that constant stirring should be employed during the process.
Another student should repeat the above procedure at room temperature except that the Alka-Seltzer® tablet should be crumbled and powdered before it is added to the water.
|
|
Plotting
|
|
|
Data Table:
|
|
|
Results:
|
When you finish taking data, plot your results clearly on the graph paper provided by
-
reading up the vertical axis until you come to your time (or whatever parameter is being plotted),
-
reading across the graph horizontally until you come to your temperature, and
-
then placing a small circle.
Note that your circle will not be on either axis!
Do you observe any trend based on the data plotted so far?
|
Go to Easy and Interesting Science Experiments for the Classroom Grades K-12
Go to the SCIBUS Main Page
Last revision to this page on March 5, 1998
Orlando Rainey, Webmaster
|